Jokubas Ziburkus
Members
- Anne Jacobson
- Blaine Cole
- Brigitte Dauwalder
- Cameron Buckner
- Dan Wells
- Diane Wiernasz
- Elizabeth Ostrowski
- Gregg Roman
- Ioannis Pavlidis
- Jason Eriksen
- Jokubas Ziburkus
- Leigh Leasure
- MariVi Tejada-Simon
- Mary Ann Ottinger
- Michael Rea
- Preethi Gunaratne
- Ricardo Azevedo
- Shishir Shah
- Steve Pennings
- Therese A. Kosten
- Tony Frankino
My laboratory is interested in the role of excitatory and inhibitory neuronal synaptic function and plasticity in normal and in pathological rodent and human brain tissue. We use a number of different techniques, including neurophysiology, neuronal network activity imaging, molecular biology, immunohistochemistry, and modeling. Here are the main areas of research currently under way in my laboratory.
Synaptic changes take place in every individual’s brain. Synaptic communication is a dynamic process that is altered during development, memory formation, and following pathological insults. Cellular substrates of these changes are reflected as long-term potentiation or depression of synapti activity. Recently we discovered that opposing action neuromodulators gate the polarity of long term plasticity, suggesting that the behavioral state of an individual has ability to alter synaptic plasticity learning rules.
Picture: Dr. Ziburkus’ oil on wood painting on which are superimposed spike timing dependent synaptic plasticity activation curves (graph adopted from Seol, Ziburkus et al, Neuron, 2006).
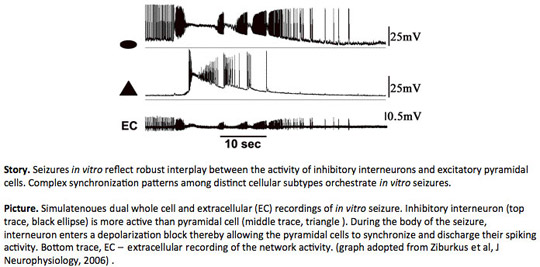
Understanding the Role of Inhibitory and Excitatory Cell Network Interactions in Epilepsy
In many normal brain phenomena, precise excitatory and inhibitory (EI) interactions dictate neuronal synchronization dynamics and shape neuronal activity. In our laboratory, we use in vitro epilepsy models and study the role of inhibition and excitation in seizure generation. Recently, using simultaneous dual and triple whole-cell recordings we discovered robust activity interplay between inhibitory horizontal oriens interneurons and excitatory pyramidal cells during in vitro seizures in rat hippocampus (Ziburkus et al., 2006). We are currently investigating the role of the other type inhibitory cell, hippocampal basket cell in the orchestration of these in vitro seizures. Additionally, we are researching how slow acting neuromodulator substances and gap junction communication affect the complex neuronal synchronization patterns during the seizures. These pharmacological manipulations will further elucidate precise cell subtype-specific contributions as well as point to cell-specific therapeutic targets for control of epilepsy.
Activity-Dependent Cell Specific Molecular Changes
Intense activity, such as observed during in vitro seizures, can very quickly change the gene network expression. Within twenty minutes of convulsant drug application, thousands of genes are upregulated while others are downregulated. Of particular interest to us are small RNA fragments called microRNAs (miRNA). There are thought to play a role in posttranscriptional modifications of small gene networks. Our lab is investigating the temporal qualities of miRNA expression during in vitro seizures.
Hormonal Modulation of Stress-Induced Synaptic Plasticity in the Locus Coeruleus
A strong connection exists between stress and the development of major depression and bipolar disorder (BPD). During stress adrenergic signaling of the brainstem nucleus Locus Coeruleus is impaired resulting in synaptic changes in amygdala, hippocampus, and neocortex. However, there is a lack of information on the synaptic modifications taking place within the LC nucleus. In collaboration with Doug Eikenburg (University of Houston, Pharmacology) we hypothesize that repetitive stress results in repetitive activation of glutamateric inputs to the LC, leading to synaptic plasticity within the LC, in the form of short and long term plasticity. Qualities of these short and long term synaptic modifications are gated by opposing action hormones, corticoid releasing factor (CRF) and norepinephrine (NE). Furthermore, downstream regulation of CRF and NE receptor activation by intracellular G protein receptor kinase 3 (GRK3) levels plays a major role in modulating G-protein coupled receptor signaling by the hormones participating in the stress response, influencing ability to adapt to stress. Thus, the goals of this study are to develop a better understanding of the unique mechanisms that contribute to the regulation of GRK3 expression within LC neurons and to investigate the role that GRK3 plays in the regulation of CRF1 and α2A-AR signaling in LC. To accomplish that, we will use in vivo learned helplessness and repeated forced swim stress model and examine the underlying changes in cellular plasticity in vitro using electrophysiology.
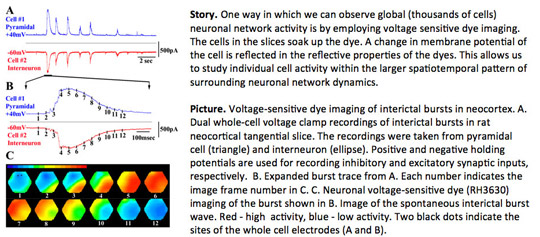
Imaging of Neuronal Activity
Concurrently with the whole-cell electrical recordings we perform voltage-sensitive dye measurements of either neuronal or glial network activity. This rare combination of techniques allows to monitor single or few cell participation in larger spatio-temporal network dynamics. Here are some fundamental questions in signal propagation in vitro that we are currently addressing: 1) What are the spatiotemporal patterns of stimulated and spontaneous waves during the early postnatal development? 2) What are the functions of distinct inhibitory and excitatory cell subtypes in normal and epileptiform neuronal waves?
Contact Us

Brigitte Dauwalder, Department of Biology and Biochemistry
University of Houston
Houston TX, 77204-5001, USA
Email: behave@uh.edu